Generics Pharma
Global Market
Oral administration segment is estimated to grow at this remarkable rate and account for the largest share of the market.
Projected to surpass ~$575 Billion by 2027
-
Total generic market growth projected to grow at a 5.6% CAGR up to 2027
-
North America dominated the generic drugs market and is projected to retain its trend
Investor News & Updates
Webinar + Presentation with Laxxon CEO Helmut Kerschbaumer – November 14, 2024
Laxxon CEO Helmut Kerschbaumer shares exclusive company updates in latest webinar from November 14, 2024.
December 20, 2023 Shareholder Update Meeting Presentation
Review the Shareholder Update Meeting Presentation from December, 20 2023
22nd Annual Needham Virtual Healthcare Conference
Our CEO Helmut Kerschbaumer is speaking at the 22nd Annual Needham Virtual Healthcare Conference.
The United States Patent and Trademark Office (USPTO) has issued U.S. Patent 11,419,824 B2 for the controlled administration of one or more active pharmaceutical ingredients within a drug delivery system via oral dosage forms.
Development of Laxxon's Levodopa Project
Our CSO Achim Schneeberger discusses progress on the Levodopa project where our technology helps us improve the quality of life for patients.

Vast End Market Opportunity
More than 150 drugs patent protections expire within the next 10 years, amounting to a total value of $190 billion in sales.
SPID®-Technology offers solutions not only to industry partners who face patent expiration but also to patients who rely on such products.
The repositioning and repurposing of drugs facilitate greater product improvement and variety, and quicker access for patients through 505B2 registrations.
Strategic Commercialization
Laxxon offers three disruptive avenues for strategic opportunities within the pharmaceutical industry:
SPID® (Screen Printed Innovation Drug) Technology
SPID® (Screen Printed Innovation Drug) Technology is Laxxon's additive 3D screen-printing and manufacturing process. With our patented technology, we can produce a generation of smart drug delivery systems (DDSs) with tailored pharmacokinetics based on advanced geometric internal structures, heterogenous distribution of active ingredients and choice of materials.
Why Invest in Laxxon?

Invest in
Laxxon
Strategic Partnerships

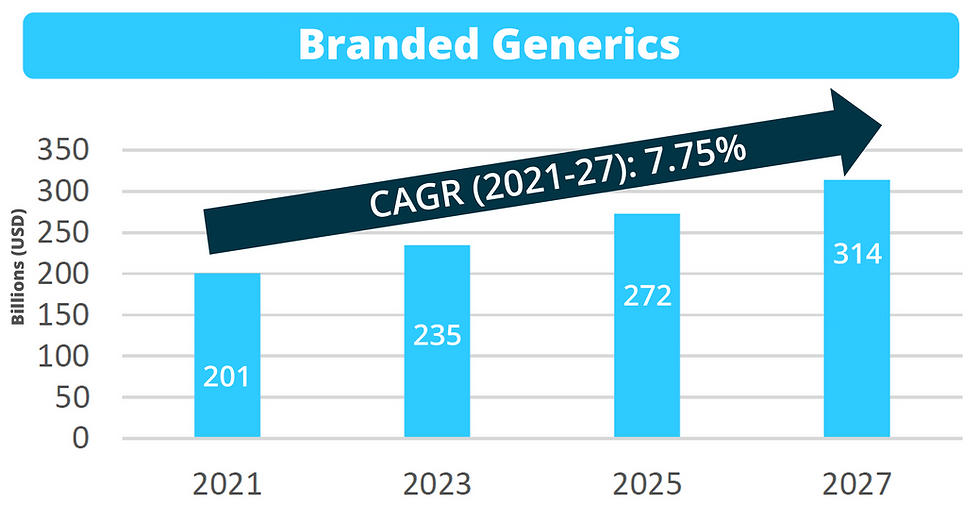
Branded generics dominated the market in 2020, but pure generics are anticipated to surpass branded generics by 2027.




Advanced Patented Generics
Through SPID®-Technology, we can repurpose, reposition, and then relaunch generic APIs with patent protection while also addressing common generic drug issues such as patient compliance, dosage, and side effects. Our Advanced Patented Generics are going to be registered in the US under the FDA Section 505(b)(2), a fast-tracked regulatory approval process. In the EU, our Advanced Patented Generics will be registered as the Hybrid applications under Article 10(3) of Directive 2001/83/EC.
Patent Extension
Laxxon offers pharmaceutical companies whose products are facing patent expiration an opportunity to "extend" their patents through Technology Transfer. Our additive approach allows for the optimization and reformulation of such pharmaceutical products which, in turn, are protected by the Laxxon IP. Our "Patent Extension" through Technology Transfer solution is for registered and safe pharmaceutical drugs on the market.

New Drug Development
Laxxon serves as a one-stop research, development, engineering, and manufacturing shop for pharmaceutical companies seeking new drug development. SPID®-Technology can produce small batch sizes for R&D and clinical studies, which can then be scaled to an industrial standard of production with no change to the manufacturing process. Laxxon offers price-aggressive manufacturing and cutting-edge, patented pharmaceutical solutions for external partners and in-house product development.

















